Impact of dietary alkalinisation on kidney’s acid excretion
Acids in the body
The acids in the human body are either ingested or metabolically produced. The kidneys, the lungs and the gut are responsible for the amount of acids in the body. In the gut, absorption of nutrients, including acids and bases, takes place. The lungs maintain the respiratory control of the body’s acid-base status (ABS) by expiring volatile acids e.g. carbonic acid. The kidneys are the only organ that can excrete the strong, non-volatile acids e.g. sulphuric acid, hydrochloric acid, etc. In addition, the kidneys regenerate bicarbonate, the major blood buffer. Thus, the kidneys have the task of regulating ABS, which with the respiratory control, maintain the blood pH in a very narrow range 7.35-7.45.
Dietary acid & alkaline sources
Fruits like oranges, apples etc. taste acidic due to organic acids they contain, such as citric acid or malic acid. Although these organic acids are acidic, they have no prolonged acidifying impact in the body because they are volatile and are usually fully metabolised and expired as carbon dioxide. Only those organic acids escaping renal reabsorption act metabolically acidic because they demand cationic bases for their excretion.
According to current knowledge, foods having an excress of inorganic anions (e.g. Cl,SO4) over inorganic cations (Na, K, Mg) are acidic and those having an excess of cations over anions are alkaline. Inorganic cations are alkaline and inorganic anions are acidic. Proteins contain sulphur, which is a source of the strong dietary acid, sulphuric acid. Such acidic anions require corresponding alkaline cations (Na, K, Mg) to buffer them, both in metabolism and excretion. Fruits and vegetables (F&V), especially potatoes, leafy greens, herbs like parsley, dried fruits (dehydration concentrates the cations) like figs, raisins, contain alkaline cations (Na, K, Mg) usually as their organic salts in excess over the acidic, non-volatile anions (Cl,SO4). As mentioned, the organic components are largely metabolised to carbon-dioxide and expired, and the cations alkalise. Thus, F&V are good sources of dietary alkalies.
Modern day diets
Nutrient density of modern day F&V is lower than those produced in preagricultural times. General advice is to consume at least five portions of F&V daily. An increase in acid load through protein consumption can be compensated by adequate F&V consumption, as in preagricultural diets. The question is if modern day diets provide enough of these alkalies? Studies show that this is frequently not the case, leading to low-grade, latent acidosis, where the blood pH is more towards the lower end of the normal pH range. To conserve the cations in the body and maintain the body’s more alkaline pH, the kidneys compensate by increasing ammonia production. Ammonia is an important component of the body’s net acid excretion and buffers protons in the urine. In addition, bone stores of cations might be mobilised to make up the lack in dietary alkalies or to neutralise increases in dietary acids. Knowledge of the foods’ acid (alkali) content would thus be useful.
Calculation of food acidity
The potential renal acid load (PRAL) is a measure of food’s acid load. An anion excess (e.g. SO4 from protein degradation) over mineral cations (K, Na, Mg) would yield a high PRAL. This can be calculated from the amount of respective minerals in foods. The table shows the major food groups’ average PRAL values.
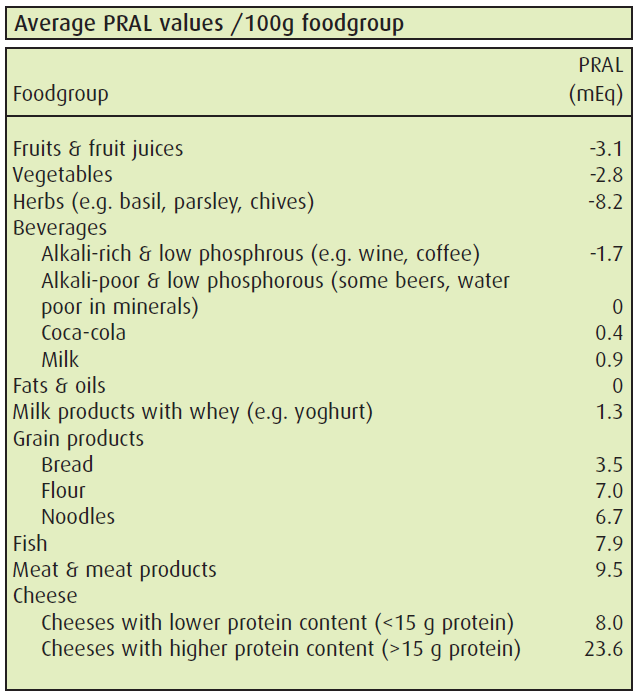
Recent studies show proteins increase bone strength in elderly and children. This is due to the anabolic effect of protein on bone. With adequate F&V consumption the protein negative effect of high PRALs is compensated. F&V ensure the availabilty of alkalies in the diet. PRAL tables help in making food choices, so that the kidney’s renal regulation is facilitated.
